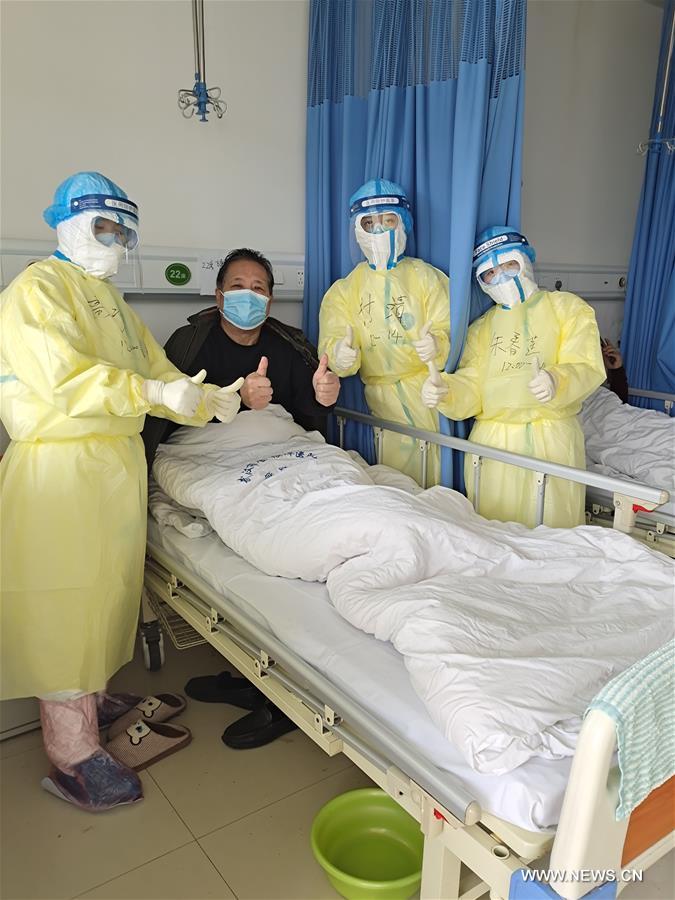
Nations join hands to find speedy cure

Promising drugs
There are two typical ways of treating a viral infection. One is using small-molecule drugs that can stop the virus from replicating by interfering with its proteins. The other way is to use antibodies that bind to the virus and cause it to self-destruct, according to the World Health Organization.
Zhong Nanshan, one of the leading experts tackling the outbreak in China, told Xinhua News Agency on Sunday that there are at least seven existing small-molecule drugs going through various stages of clinical trials in China.
On Sunday, Beijing's China-Japan Friendship Hospital announced it will begin clinical trials on 270 mildly and moderately ill patients infected with the novel coronavirus in the epicenter of the outbreak in Central China using an experimental drug from the US called remdesivir, which was originally developed as an Ebola cure.
China's Center for Drug Evaluation of the National Medical Products Administration approved the trials on Sunday to be carried out in Wuhan, Hubei province, from Monday to April 27, its official website said.
The drug is developed by US biotech company Gilead Sciences. It was provided on compassionate grounds to a 35-year-old man in the US infected with the novel coronavirus, and his symptoms noticeably improved within a day with no obvious side effects, according to a paper published in the New England Journal of Medicine last week.
The US drugmaker said in an online statement last week that it is working closely with health authorities in China and around the world to respond to the novel coronavirus outbreak.
However, it stressed that remdesivir has "not yet been licensed or approved anywhere globally and has not been demonstrated to be safe or effective".
While there is no data regarding remdesivir's effectiveness against the novel coronavirus, the drug has demonstrated antiviral capability in animal models against viral pathogens like SARS and MERS — fellow coronaviruses that are structurally similar to the one from Wuhan, the company said.
Qian Jiahua, chief scientist at the drug evaluation center, told Chinese science news outlet Intellectual that the clinical trial period in China can be shortened if remdesivir produces the same impressive results seen in multiple test patients.
"I hope the drug is like penicillin and can have an immediate impact on a disease," Qian said. While Qian lauded the joint effort, he highlighted the fact that the drug is not yet registered in China and Chinese scientists don't know much about its clinical test results done in the US.
"I think we should move with caution before mass administrating it to a demographic as diverse and complex as the Chinese people," he said.
Zeng Weigen, a doctor at Beijing Chaoyang Hospital, said remdesivir has proved to be effective on one US patient with symptoms of pneumonia, but it will require more clinical trials to confirm its potency against the novel coronavirus.
"In terms of safety, this drug has been thoroughly tested on humans, mainly on Ebola patients, and its side effects are not that pronounced," Zeng said.
"We hope it can have a good inhibiting effect on the virus and save our Chinese patients. We're looking forward to more data from clinical trials," he added.
Apart from remdesivir, scientists are also testing a cocktail of flu and HIV drugs that had showed potential in curbing the coronavirus. Thailand's Ministry of Health said on Sunday that Thai doctors have seen apparent success treating a 71-year-old woman infected with the novel coronavirus.
The doctors used a combination of the flu drug oseltamivir with lopinavir and ritonavir — antivirals used to treat HIV. The patient's health significantly improved and she tested negative for the virus 48 hours after Thai doctors administered the combination.
However, doctors are still monitoring the patient and waiting for scientists to prove their findings. Hospitals in Beijing have reportedly been using the same HIV drugs as part of treatment for the novel coronavirus, though it is unclear if they have been successful.
Zhang Dingyu, president of Wuhan Jinyintan Hospital, said at a news briefing on Sunday that HIV drugs like lopinavir have kept severely ill patients at his hospital from worsening.
However, Zhang said such drugs have some serious side effects including diarrhea, allergic reactions, liver damage and slowed heart rates, and must be taken under close supervision and guidance.
- Beijing explores robots to support aging population
- Aid cadre decides to stay permanently in Xizang
- Inner Mongolia's two ports named national smart port models
- Ministry lodges stern representations with US over $11b arms sales plan to Taiwan
- Unlocking life in Gansu: an Egyptian professor's journey with the Five-Star Card
- NE China's Heilongjiang issues highest-level blizzard warning