Quality Chinese vaccines keep nation's promise
Domestic pharma companies work overtime to meet soaring demand at home and abroad

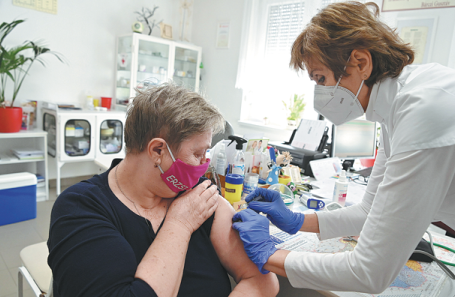
During this year's Feb 11-17 Spring Festival holiday-the most important annual cultural event for Chinese-more than 1,200 employees of Sinovac Life Sciences, a pharmaceutical company based in Beijing, did something unprecedented.
Unlike hundreds of millions of fellow citizens who tried to celebrate the holiday in innovative ways this year due to restrictions relating to the COVID-19 pandemic, Sinovac staff members sacrificed festivities and continued working.
That enabled Sinovac to function round the clock and produce hundreds of thousands of doses of CoronaVac vaccine, an inactivated COVID-19 vaccine. The company's plants operated at full capacity to meet the soaring demand at home and abroad.
As of March 3, COVID-19 has spread to more than 223 countries and regions, with about 114.43 million confirmed cases, and more than 2.54 million casualties, according to the World Health Organization.
Those figures also underscored the widening immunity-development gap between the world's high-income countries and those underprivileged, which has resulted in a global vaccine accessibility inequity, adding uncertainties to hopes that life could return to normal through vaccinations.
About 97 countries and regions have administered more than 208 million doses of COVID-19 vaccines as of Feb 23. Richer countries such as the United States took leading places in terms of population fully or partially vaccinated, according to Our World in Data, an online research provider affiliated with the Oxford Martin Programme on Global Development.
To bring the pandemic to an end, the world's population needs to become immune to the novel coronavirus-SARS-CoV-2-through vaccinations. China has been unrelenting in its efforts to strengthen the global virus fight.
For instance, it has invested time, energy and big money on vaccine development and production, and boosted vaccine exports to countries in dire need. China has also championed the cause of making COVID-19 vaccines a global public good-something that Premier Li Keqiang underscored in the Government Work Report delivered at the fourth session of the 13th National People's Congress on Friday.
The report said: "We upheld multilateralism and endeavored to build a human community with a shared future. We supported global cooperation on combating COVID-19 and called for building a global health community. China thus made important contributions to advancing global peace and development."
"Important" here is borne out by hard evidence. Guo Weimin, spokesperson for the fourth session of the 13th National Committee of the Chinese People's Political Consultative Conference, the country's top political advisory body, said on Wednesday that as of Feb 28, China has offered COVID-19 vaccine assistance to 69 countries and two international organizations, and exported vaccines to 28 countries. Guo also said these numbers are growing and it is "very narrow-minded" to perceive China's action of providing COVID-19 vaccines and related assistance to other countries as a tool for gaining geopolitical influence.
China has pledged to provide vaccines as a global public good, and encourages Chinese companies to collaborate with other countries when developing and producing vaccines, he said.
Zhang Yesui, spokesperson for the fourth session of the 13th National People's Congress, said on Thursday that China had 17 vaccine candidates entering clinical trials, with several already in third stage clinical tests.
The National Medical Products Administration has given conditional approval for four homegrown vaccines developed by China National Pharmaceutical Group, which is known as Sinopharm, Sinovac, and CanSino Biologics Inc.
According to Feng Duojia, president of the China Association for Vaccines, China's annual COVID-19 vaccine production capacity is expected to be more than 2 billion doses by the end of this year, and can grow to more than 4 billion doses by the end of 2022, if necessary.
"Chinese vaccine producers are building an increasingly greater presence in the world, as reflected by their exports of COVID-19 vaccines, to contribute to global containment of the pandemic," he said.
"Chinese companies are also furthering their efforts for production efficiency improvement and cost control, to help ensure fair distribution of the vaccines as a global public good among developing countries."
More than 20 countries have signed supply contracts with China for the vaccines, with orders from 16 countries and regions already exceeding 50 million doses, he said.
"We will try our best to produce more high-quality vaccines as quickly as possible on the basis of safety and standards to contribute to fairer distribution of COVID-19 vaccines," said Yang Xiaoming, chairman of China National Biotec Group (CNBG), a subsidiary of Sinopharm, and a major COVID-19 vaccine producer in China.
It is said all people are susceptible to COVID-19. Countries around the world should work together on vaccination, personnel protection, and community management to effectively control the disease, Yang told Xinhua News Agency.
China's decision to supply 10 million doses of COVID-19 vaccine to the WHO's global vaccine sharing initiative COVAX signals that the country has honored its commitment to make the vaccines a global public good, he said.
Sinopharm's annual production capacity has already hit 1 billion doses. About 60 million doses of its vaccines have been administered worldwide, Yu Qingming, chairman of Sinopharm Group Co Ltd, a subsidiary of Sinopharm, told the Beijing-based Health Times.
"Currently, most of the countries that received vaccines from China are developing countries, which basically are not capable of independently developing or producing COVID-19 vaccines, and are in dire need of the vaccines compared with other countries," Yang of CNBG said.
The Sinovac vaccine has been approved for emergency use in several countries, including Indonesia, Brazil and Chile, according to Yin Weidong, chairman and CEO of Sinovac Biotech Ltd.
At the end of February, the company had finished its capacity expansion project to increase annual production capacity to 1 billion doses.
"Sinovac has received vaccine orders from Brazil, Indonesia, Turkey, Chile and some other countries and regions, and we are making every effort to expand the production capacity," Yin said.
Pearson Liu, director of brand management and public relations of Sinovac, said the company will export semifinished jabs to some countries, and help build local filling and packaging lines in importing countries to improve the production capacity and efficiency.
The company's COVID-19 vaccine exported overseas received warm welcome in destination countries.
On Jan 14, Turkish President Recep Tayyip Erdogan received the COVID-19 vaccine produced by Sinovac.
In Indonesia, the president, cabinet members, as well as influential public figures in the country, received Sinovac's vaccination.
Apart from the four approved vaccines, vaccine candidates under development by other Chinese producers are making steady progress. For instance, the mRNA vaccine developed by Walvax Biotechnology Co Ltd and its partners, is said to be a strong candidate.
Walvax, the only domestic producer of China's self-developed 13-valent pneumococcal polysaccharide conjugate vaccine, said it has three COVID-19 vaccine candidates on its hands. Among them is the mRNA vaccine, which uses a new technology to stimulate the immune system to trigger appropriate responses against the virus.
The mRNA vaccine received approval from the NMPA for clinical trials on June 19, and has already entered phase-II clinical trials. It also has excellent stability in storage at 2-8 C, which means it will be much less demanding during transportation and in terms of storage conditions, compared to similar mRNA vaccines developed by other producers.
The company has started the construction of a manufacturing base with considerable capacity for the mRNA vaccine, which is expected to reach production in a few months.

Today's Top News
- Cultural events marking victory against Japanese aggression unveiled
- Wang Yi holds talks with Belgian FM Maxime Prevot
- European Commission president meets with Wang Yi
- Beijing calls for enhancing communication with EU
- Ministry warns of flood risk in north, drought in south
- Green energy powers Indonesia ties






























