Gardasil is the second such drug to gain access to patients across China
Pharmaceutical company Merck recently won approval from the China Food and Drug Administration to sell its human papillomavirus vaccine, Gardasil, to help women fight cervical cancer.
Developed by the US-based company in 2006, the vaccine has proved effective in protecting against the virus, better known as HPV, the chief cause of cervical cancer. The virus is found in almost all cervical cancer cases.
Gardasil is the first HPV vaccine in the world and the second to be licensed for use in China.
In July, Cervarix, an HPV vaccine developed by pharmaceutical GlaxoSmithKline, received approval to be sold on the Chinese mainland after almost 10 years of seeking approval.
Gardasil is expected to be commercially available on the mainland in three to six months, which means women will no longer have to seek vaccinations outside of the mainland, such as Hong Kong.
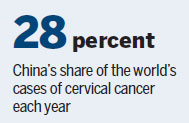
After breast cancer, cervical cancer is the second-most common cancer in women ages 15 to 44 in China. Statistics from the Chinese Center for Disease Control and Prevention show China reports more than 130,000 cervical cancer cases a year, accounting for 28 percent of global total.
The HPV vaccine, as the first anti-cancer vaccine in the world, has proved effective in preventing cervical cancer and is seen as a breakthrough in the fight against cancer.
Gardasil offers protection against nine strains of HPV, including the two main cancer-causing varieties: type 16 and type 18. Cervarix offers protection only against types 16 and 18, which account for about 70 percent of all cervical cancer cases.
Today, such vaccines are used in about 120 countries and regions, including the United States, Australia and most European countries.
As HPV is sexually transmitted, the World Health Organization recommends routine vaccination of girls age 9 to 13 because they are not as likely to have begun sexual activity.
Qiao Youlin, a professor of epidemiology at the Chinese Academy of Medical Sciences' Cancer Hospital in Beijing, said the main target group of Cervarix is females age 9 to 26, although it is theoretically effective for women of all ages.
Clinical trials discovered the vaccine is effective for women as old as 45, he said.
According to the Securities Times newspaper, Zhifei Biological Products Co in Chongqing will promote, supply and sell the vaccine in China for the next three years.
As the exclusive distributor of the vaccine, the company plans to purchase 1.14 billion yuan ($166 million) worth of Gardasil vaccines in the first year, 1.48 billion yuan in the second year and 1.85 billion yuan in the third year, the newspaper said.
Shan Juan and Yang Wanli contributed to this story.
zhaoxinying@chinadaily.com.cn
